Unexplained transient loss of consciousness after legionella pneumonia
Nynke van Dijk, C.T. Paul Krediet and Wouter Wieling, Department of Medicine, Academic Medical Centre. University of Amsterdam, Amsterdam (The Netherlands)
A 58 year-old male was referred by a cardiologist to our syncope unit for evaluation of an unexplained episode of transient loss of consciousness. Four years prior to this visit he had been admitted to an intensive care unit for a Legionella pneumonia. On recovery urinary bladder dysfunction and erectile dysfunction were evident. An urologist diagnosed prostate hypertrophy and a Trans Urethral Resection of the Prostate (TURP) was performed. The problems of the sensation of a full bladder and frequent voiding (6 to 8 times at night) remained. Urinary incontinence was not present. Urodynamic evaluation showed an atonic areflexic bladder. Self-catheterisation was started. Prior to the Legionella pneumonia his general health and sexual and bladder functions had been normal. In the years following this episode the patient experienced progressive problems of fatigue and dyspnea during stair climbing. These symptoms were attributed to diminished pulmonary function after the Legionella pneumonia. He also complained of light-headedness during prolonged standing and near-syncope during toilet visits at night. A few months prior to the visit to our unit he lost consciousness on a very hot day shortly after standing up from a chair. He fell into a swimming pool and almost drowned. After this episode cardiological assessment was performed An EKG and echocardiogram both were normal. During tilt-table testing a large drop in blood pressure was observed after administration of nitroglycerin (from 150/80 mm Hg prior to the tilt test to 60 mmHg systolic during the test), but the patient did not faint and the test result was interpreted as negative. During bicycle exercise testing the patient had to stop at a load of 120 Watt only, because of fatigue and light-headedness. Blood pressure dropped from 150/60 mmHg sitting on the bicycle to 60/40 mmHg directly post-exercise. No EKG-changes were noted. Because the fall in blood pressure during exercise was unexplained, cardiac catheterisation including ventricular and coronary angiography was performed to exclude cardiac disease. No abnormalities could be found.
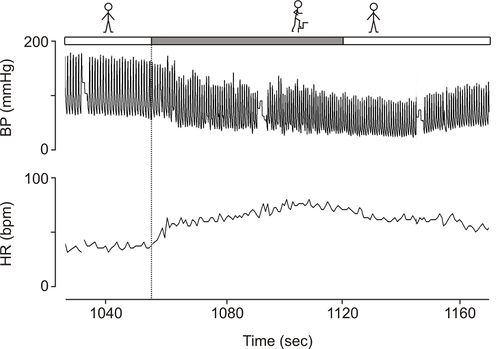
On examination in our syncope unit he was in good general health. His finger arterial pressure was 190/93 mmHg supine with an asymptomatic fall to 175/93 after 3 min standing. During an exercise test (step-test) blood pressure decreased in 1 minute from values around 175/95 mmHg to levels of 114/64 (Figure 1).
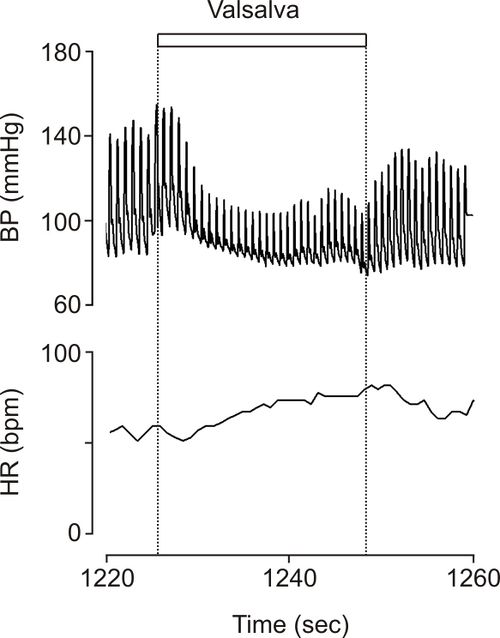
During a Valsalva test there was a large fall in blood pressure with hardly any recovery in pressure during straining and no overshoot of blood pressure after release of the strain (Figure 2). Heart rate followed the changes in blood pressure suggesting intact afferent, central and efferent cardiac arterial baroreflex pathways. The lesion therefore appeared to be located in the efferent sympathetic vasoconstrictor pathways.
Based on the symptoms of orthostatic hypotension and abnormalities in urinary bladder and sexual function autonomic neuropathy was diagnosed clinically and confirmed by autonomic testing. Autonomic neuropathy has been described as a complication of a Legionella infection earlier [1]. The patient received explanation and instructions about his condition (see case 5 in this section).
Editor's comments
Lightheadedness induced by exercise is a recognized presenting feature of ischemic heart disease [2]. The referring cardiologist, therefore, was puzzled when he was unable to demonstrate a cardiac abnormality. However, an adequate rise in blood pressure during exercise is not only dependent on normal cardiac function, but also on redistribution of bloodflow. Shunting of oxygenated blood to active muscles does not only result from decreased vascular resistance in active skeletal muscles, but in particularly from strong sympathetic mediated vasoconstriction in non-exercising skeletal muscle and splanchnic and renal vascular beds. Vasoconstriction of these tissues is a prerequisite to obtain an adequate physiological blood pressure increase to exercise. Vasoconstriction, however, is blunted in patients with autonomic neuropathy [3].
Autonomic neuropathy is a rare disorder, but the presentation in this patient is classical [4]. Hypotension during common daily activities in patients with autonomic failure was recognized by earlier investigators. Climbing stairs in particular was noted to elicit symptomatic hypotension [5][6]. The complaints during stair climbing in our patient were at first attributed to his diminished pulmonary function, but laboratory testing disclosed that stair climbing induced symptomatic hypotension [Fig. 1]. The absence of sympathetic vasoconstrictor activity in non-active skeletal muscles and other vascular beds , is not compensated for by the increase in cardiac output that occurs during dynamic exercise [7]. As a consequence, blood pressure falls even if the exercise is performed supine, and, as the patient develops muscle fatigue and stops exercising, a further drop in blood pressure occurs [8][9][10]
Other frequently reported complaints in patients with autonomic failure are (pre)syncopal symptoms during toilet visits at night and in the early morning [2][3]. This complaint also was present in our patient.
In conclusion, in patients with (pre)syncope during exercise combined with miction, defecation, sweating or erectile problems in the absence of cardiac disorders, autonomic dysfunction is likely to be present and autonomic evaluation, including exercise testing and a Valsalva manoeuvre should be performed. By just testing of blood pressure in supine and standing position the diagnosis might be overlooked [9].
References
-
Bernardi DL, Lerrick KS, Hoffman K, Lange M. Neurogenic bladder. New clinical finding in Legionnaires’ disease. Am J Med 1985; 78: 1045-1046.
- Weiner DA, McCabe CH, Cutler SS, and Ryan TJ. Decrease in systolic blood pressure during exercise testing: reproducibility, response to coronary bypass surgery and prognostic significance. Am J Cardiol. 1982 May;49(7):1627-31. DOI:10.1016/0002-9149(82)90238-7 |
-
Rowell LB. Human cardiovascular control. Oxford, Oxford University Press 1993.
-
Bannister R, Mathias CJ. Management of postural hypotension. In: Mathias CJ, Bannister R. Autonomic Failure. A Textbook of clinical Disorders of the Autonomic Nervous system. Oxford, Oxford University Press 1999 pp 342-356.
-
Bradbury S, Eggleston C. Postural hypotension: A report of three cases. Am Heart J 1925; 1: 73-86.
- Wieling W, van Lieshout JJ, and van Leeuwen AM. Physical manoeuvres that reduce postural hypotension in autonomic failure. Clin Auton Res. 1993 Feb;3(1):57-65. DOI:10.1007/BF01819146 |
- Rowell LB. Neural control of muscle blood flow: importance during dynamic exercise. Clin Exp Pharmacol Physiol. 1997 Feb;24(2):117-25. DOI:10.1111/j.1440-1681.1997.tb01793.x |
-
Marshall RJ, Schirger A, Shepherd JT. Blood pressure during supine exercise in idiopathic orthostatic hypotension. Circulation 1961; 24: 76-81.
-
Smith GD, Bannister R, Mathias CJ. Post-exertion dizziness as the sole presenting symptoms of autonomic failure. Br Heart J 1993;69:359-61.
-
Krediet CT, Wilde AA, Wieling W, Halliwill JR. Excercise related syncope: when it’s not the heart [review]. Clin Auton Res 2004; 14 Suppl 1:25-36.