Sandbox
Triggers for Vasovagal Syncope
Vasovagal syncope can occur after exposure of a lot of different triggers. Recognised triggers for vasovagal syncope are prolonged orthostatic stress, blood drawing, medical instrumentation and psychological stressors.
Psychological stressors
Psychological stressors include:
- Stirring emotional news or witnessing a distressing accident [1],[2]
- Unexpected pain or threat [1],[3]
- Unpleasant smells may trigger vasovagal syncope [4],[5]
- During blood drawing,
- Vaccination [6]
- Instrumentation, pain of the procedure may contribute to vasovagal syncope
- Sharp pain is reported to be an important factor during arterial blood sampling [7].
- Blood phobia: However, in a patient with blood phobia just thinking or talking about blood drawing may elicit a common faint [8]
Post-exercise vasovagal syncope
Syncope after exercise is often neurally mediated, i.e. post-exercise vasovagal syncope. This condition is typically diagnosed in young fit, furthermore healthy young patients.
Foremost, the diagnostic workup of all patients presenting with exercise-related syncope is aimed at excluding dangerous cardiac conditions and includes echocardiography and exercise testing [9]. Risk factors for a cardial problem are fainting while sitting or supine and suddenly fainting during exercise without presyncope.
Characteristically, syncope may occur while the individual is standing motionless during the first five to ten minutes after exercise [10]. Especially athletes in the (ultra) endurance sports are at risk for post exercise vasovagal syncope e.g. after marathon swimming [11] or marathon running [12][13][14].
Vasovagal syncope after routine treadmill testing is rare (estimated 0,2% [15]). However, when treadmill testing is immediately followed by passive head-up tilt testing, this percentage can increase up to 50-70% [10].
Vasovagal syncope after exercise is considered to be a benign occurrence [9].
Muscle pump
During exercise, rhythmically contracting skeletal muscles in the lower part of the body reduce the degree of venous pooling by squeezing veins, thereby increasing the venous return of blood to the heart. This phenomenon is known as the ‘muscle pump. The sudden removal of the muscle pump after stopping exercise decreases cardiac preload which, together with a rapid return of vagal tone, may promote vasovagal syncope.
Vasovagal syncope in airliners
Vasovagal episodes are the most common in-flight medical events, and may affect patients of all ages [16].
The following may all predispose vasovagal faints during air travel [17]:
- Prolonged motionless sitting
- The use of alcohol
- Anxiety
- Mild hypoxia during air travel
Cabin pressure in commercial aircraft is usually adjusted to the equivalent of an altitude of 1500 to 2500 m above sea level. It appears that hypoxic syncope results from the super-imposed vasodilator effects of hypoxia on the cardiovascular system [18].
Treatment
Patients, who otherwise never experienced a (severe) vasovagal episode may suffer from convulsive syncope during air travel [19]. These patients should be advised to have:
- A high salt intake in the days prior to travelling by plane
- Reduce anti-hypertensive medication –if feasible-
- And drink non-alcoholic beverages galore during the trip.
Especially during long flights (> 2 hours) they should perform in-chair muscle tensing and relaxing exercise and have a regular walk through the isle. In recurrent cases midodrine prior to flying or supportive stockings can be considered.
Sleep vasovagal syncope
Sleep vasovagal syncope is defined as loss of consciousness in a non-intoxicated adult occurring during the night (e. g. 10:00 pm to 7:00 am), in which the patient wakes up with pre-syncopal and abdominal symptoms (i.e. an urge to defecate) and losses consciousness in bed or immediately upon standing.
There is no tongue biting or post-ictal confusion. There is usually a history of daytime vasovagal syncope and there seems to be a more pronounced fear of blood and medical procedures than in other syncope patients [20]. Physical examination, ECG and EEG are within normal limits. The vasovagal reaction is thought to start while asleep [21][22], and continuing after waking up, hence the name. During syncope there may be a profound sinus-bradycardia [21]. Vasovagal sleep syncope occurs at all ages.
Differential Diagnosis
Sleep vasovagal syncope is diagnosed by excluding beyond reasonable doubt the hereafter mentioned disorders [22].
Epilepsy is the foremost alternative diagnosis to consider, but can often easily be ruled out on clinical grounds. Complex partial, generalized tonic-clonic and myoclonic epilepsy may occur during sleep and can imitate syncope when causing cause sinus-bradycardia [23].
There are a number of related conditions, including “abdominal epilepsy” and Panayiotopoulos syndrome (typically with vomiting) [24], in which the associated clinical features are abdominal pain and confusion.
Sleep paralysis and hypnogogic hallucinations occur in narcolepsy but also as isolated phenomena, mostly with other characteristic features in the history (e. g., daytime somnolence, in contrast to syncope there’s no amnesia.) and abnormal polysomnography, which can also be used to diagnose sleep apnoea and night terrors.
Occasionally cardiac disorders may cause cardiac arrhythmias during sleep. Most of these are unlikely if the 12-lead ECG is normal, and in some patients long-term ambulatory ECG monitoring is required [25].
Some patients with a diagnosis of defaecation syncope (see below) described abdominal and pre-syncopal symptoms that started simultaneously during sleep [26][27] there may be some overlap between this condition and sleep syncope [22].
References
Error fetching PMID 12686351:
Error fetching PMID 9080932:
Error fetching PMID 11227958:
Error fetching PMID 15480927:
Error fetching PMID 6650203:
Error fetching PMID 8531620:
Error fetching PMID 52682:
Error fetching PMID 8614313:
Error fetching PMID 9397327:
Error fetching PMID 4800477:
Error fetching PMID 11932475:
Error fetching PMID 11543489:
Error fetching PMID 15531565:
Error fetching PMID 16836688:
Error fetching PMID 15084573:
Error fetching PMID 11701591:
Error fetching PMID 16950946:
Error fetching PMID 11445105:
Error fetching PMID 83099:
Error fetching PMID 264159:
- Error fetching PMID 20776843:
-
Engel GL, Romano J, & McLin TR (1944). Vasodepressor and carotid sinus syncope - clinical, eletroencephalographic and electrocardiographic observations. Arch Intern Med 74, 100-119.
-
GREENFIELD AD (1951). An emotional faint. Lancet 1, 1302-1303.
-
Engel GL & Romano J (1947). Studies of Syncope: IV. Biologic interpretation of vasodepressor syncope. Psychosom Med 9, 288-294.
- Error fetching PMID 12686351:
- Error fetching PMID 9080932:
-
Rushmer, R.F. Circulatory collapse following mechanical stimulation of arteries. Am. J. Physiol. 1944;141:722.
- Error fetching PMID 11227958:
- Error fetching PMID 15480927:
- Error fetching PMID 6650203:
- Error fetching PMID 8531620:
- Error fetching PMID 52682:
- Error fetching PMID 8614313:
- Error fetching PMID 9397327:
- Error fetching PMID 4800477:
- Error fetching PMID 11932475:
- Error fetching PMID 11543489:
- Error fetching PMID 15531565:
- Error fetching PMID 16836688:
-
Jardine DL, Krediet CT, Cortelli P, & Wieling W (2006b). Sleep syncope: clinical features and autonomic profiles. Clin Auton Res 16, 321-322.
- Error fetching PMID 15084573:
- Error fetching PMID 11701591:
- Error fetching PMID 16950946:
- Error fetching PMID 11445105:
- Error fetching PMID 83099:
- Error fetching PMID 264159:
Initial Orthostatic Hypotension
Initial orthostatic complaints originate from a transient rapid fall in arterial pressure occurring upon active standing. This fall in blood pressure is a physiological response (Sprangers et al., 1991). However, normally blood pressure does not drop for more than 40 mm Hg systolic and 20 mm Hg diastolic (See chapter Wieling & Karemaker). The onset of symptoms between 5-10 s and disappearance within 20 seconds after the onset of standing up is typical for this condition. The diagnosis can only be confirmed by a stand test with continuous beat-to-beat blood pressure monitoring (figs. 5 and 6)(Wieling et al., 2007). Because initial orthostatic hypotension is associated with ‘active’ arising (fig. 5), tilt testing (i.e. head up tilting) wil not reveal a diagnosis.
Epidemiology
Most teenagers and adolescents are familiar with a brief feeling of light-headedness and some visual blurring within a few seconds of standing up quickly (de Marées, 1976). Symptoms typically resolve spontaneously within 20 s. Such complaints are most common upon arising suddenly after prolonged supine rest or after arising from the squatted position (figs. 5 and 6) (Krediet, 2002;Wieling et al., 2007). In some the symptoms are severe and syncope may occur upon standing in otherwise healthy subjects (Wieling et al., 2007). In a study, performed in 2003, in 394 young adults (i.e. medical students) standing up was reported as the trigger for transient loss of consciousness in 8% (Ganzeboom et al., 2003).
Triggers
Rising from squatting encompasses a heavier orthostatic stress than rising from supine (SHARPEY-SCHAFER, 1956;Rossberg & Penaz, 1988). On average blood pressure in healthy young adults falls transiently by 60 mm Hg systolic and 40 mm Hg diastolic with a nadir about 7 s after rising (Rossberg & Penaz, 1988). Mild symptoms of transient light-headedness are often present. Rising from squatting is a recognized trigger for syncope in daily life (fig. 6). Arising after prolonged squatting during gardening and other house-hold activities is a common scenario (SHARPEY-SCHAFER, 1956).
Treatment
Treatment of initial orthostatic hypotension is symptomatic. The goal is to diminish the drop in blood pressure after standing up. A clear explanation of the underlying mechanism and avoidance of the main triggers (rapid rise) are the main treatment-options. A novel approach is training in blood pressure rising manoeuvres. Tensing of leg, abdominal and buttock muscles for 20-40 s at maximal voluntary force immediately after standing up may be an effective manoeuvre to decrease the fall in pressure (Krediet & Wieling, 2004). In addition volume-expansion can be applied by raising water- and salt-intake (Shichiri et al., 2002;Wieling et al., 2004a).
Carotid Sinus Syndrome
Carotid sinus hypersensitivity was defined in the 1930s. The definition included the following in respons to carotid sinus massage for 5-10s:
- asystole for more than 3s (cardio-inhibitory type) or
- systolic blood pressure fall of 50 mmHg (vasodepressor type)
- or both (mixed type)
Carotid sinus hypersensitivity is almost exclusively diagnosed in patients over 50 years of age.
In the older literature the clinical presentations of this disorder are reported to be heterogeneous. In the vasodepressor type patients would be likely to have a prodromal pattern as in classical vasovagal syncope, whereas the cardio-inhibitory type could occur without warning and present as a clinical Adams-Stokes attack. In the latter, on regaining consciousness typically there is a facial flush (FRANKE & BRACHARZ, 1956). However even under standardized laboratory conditions both types often can not be distinguished on clinical grounds.
Triggers
The prevalence of spontaneous carotid sinus syndrome induced by every-day manipulations like wearing a tight collar, shaving, head turning or stretching the neck is unknown, but likely to be rare, since its occurrence is reported as only 1% of causes of syncope in clinical settings (for review see (Colman et al., 2004a)). However in a series of 33 cases of carotid sinus hypersensitivity (among a total of 130 consecutive syncope patients) head-turning as a trigger was reported in 52% of the cases (Kenny & Traynor, 1991). Most syncopal episodes attributed to carotid sinus hypersensitivity after laboratory testing occur apparently spontaneously.
Epidemiology
The prevalence of carotid sinus hypersensitivity (i.e. a positive test in a syncope patient without a typical history of loss of consciousness following neck manipulation) in the general population is between 1 and 25%, occurring primarily in older patients, with a strongly positive correlation with age (Humm & Mathias, 2006). However since the reflex can also be triggered in otherwise healthy elderly without a history compatible with the carotid sinus syndrome (i.e. falls, dizziness and light-headedness) (Humm & Mathias, 2006;Kerr et al., 2006) the true clinical importance of carotid sinus hypersensitivity remains unclear.
Treatment
Cardiac pacing is the therapy of choice in syncope patients with documented asystole (>3 s) in response to carotid sinus massage, or on ambulatory ECG recording (Kenny et al., 2001). The vasodepressor type is likely to benefit from general orthostatic tolerance enhancing measures such as salt and volume loading, and there is some evidence that fludrocortisone may be effective (Hussain et al., 1996). However up to date there are no large scale clinical trials confirming this.
Swallow Syncope
Swallow syncope incorporates two separate conditions:
- a pharyngeal form which is usually associated with pain (“syncopal glossopharyneal neuralgia”)
- an oesophageal variety, also known as “oesophageal” or “deglutition syncope” (fig. 7)(Basker & Cooper, 2000).
The first isolated case description dates to 1906 (Mackenzie, 1906). Based on the fact that up to date there are only about sixty single cases reported (DEUCHAR & TROUNCE, 1960;Basker & Cooper, 2000), swallow syncope is probably rare.
The clinical presentation of glossopharyngeal neuralgia is a patient with (seemingly) spontaneous overwhelming pain in a jaw, tongue or throat. In some patients this pain can be triggered by touching a certain spot in the oral cavity, or by talking or swallowing. Among all patients with glossopharyngeal neuralgia, syncope seems a rare complication (Rushton et al., 1981).
In the oesophageal variety syncope typically occurs during or shortly after swallowing. Several reports point especially to cold drinks as the culprit (Rainford, 1975;Brick et al., 1978;Olshansky, 1999). Syncope triggered by belching has been reported (Kim et al., 2005).There are some that report that mention retro-sternal pain induced by swallowing as a trigger. Several cases report oesophageal syncope during recovery from myocardial infarction. Swallowing syncope is characterised by a slow or absent pulse.
Pathophysiology
The pathophysiology of both entities is poorly understood. The possible neurological pathways and mechanisms that might be involved in swallow syncope cannot be explained on the basis of any known normal reflex (Basker & Cooper, 2000). Oesophageal syncope is often ascribed to a vagovagal reflex (i.e. both afferent and efferent stimulus through the vagus nerve)(Piek et al., 1988); however this merely describes part of the peripheral functional anatomy.
In many cases of oesophageal syncope functional, endoscopic and radiological studies reveal oesophageal abnormalities including diverticulum, hiatal hernia, achalasia, stricture and neoplasms (Basker & Cooper, 2000). Although such co-morbidity may be over-reported, in the total clinical picture these case reports may be an argument to commence an in depth gastro-oesophageal evaluation.
Treatment
There are several therapeutic options.
- First, in case of overt oesophageal lesions, these should be treated.
- Secondly, if feasible, modification of antihypertensive drugs should be considered.
Surgical or pharmacological destruction of (the oesophageal branches of) the vagus nerve has shown not to be successful. Based on predominantly positive therapeutic response in over a dozen cases, the treatment of choice of oesophageal syncope is insertion of a cardiac demand pacemaker (Basker & Cooper, 2000). Management of persisting neuralgia is symptomatic.
Arterial system
The arterial system consists of three major groups of arteries. These are:
- Elastic arteries
- Muscular arteries
- Arterioles
Elastic arteries
Elastic arteries are the aorta and the major branches of it close to the heart. The vessel walls contain the most elastin of all vessels and elastin is found in all the vessels layers. Elastic arteries also contain smooth muscle cells, but these are mostly inactive in vasoconstriction. By elastically dilating when blood pressure increases (right after a heartbeat) and contracting when pressure drops they maintain blood flow in between heartbeats. The elasticity also gives a more constant BP throughout the rest of the vascular system. The diameter of elastic arteries ranges from 2.5 to 1 cm.
Muscular arteries
Muscular arteries have a diameter of 1 to 0.3 cm and derive from elastic arteries. Muscular arteries regulate the blood flow to specific organs. They are active during vasoconstriction and contain relatively the most smooth muscle cells in the tunica media. They contain less elastin than elastic arteries and are thus less distensible.
Arterioles
The smallest arteries are called arterioles. The tunica media of the arterioles mostly contain smooth muscle cells. Their diameter ranges from 0.3 cm to 10 µm, and the smallest arterioles lead into capillary beds. Blood flow into capillary beds is regulated by these arterioles. The arterioles change in diameter due to neural, hormonal and local chemical influences. These changes in diameter lead to the changes in blood flow in the capillary beds. When arterioles constrict, the capillary beds that lay behind the constricting arterioles is bypassed. When they dilate, blood flow in the capillary beds increases dramatically.
Capillaries
Capillaries are no arteries. They are the smallest of all blood vessels and their function is to exchange materials with every cell in the body. The average capillary diameter is 8 to 10 µm, so that red blood cells can just pass through them.
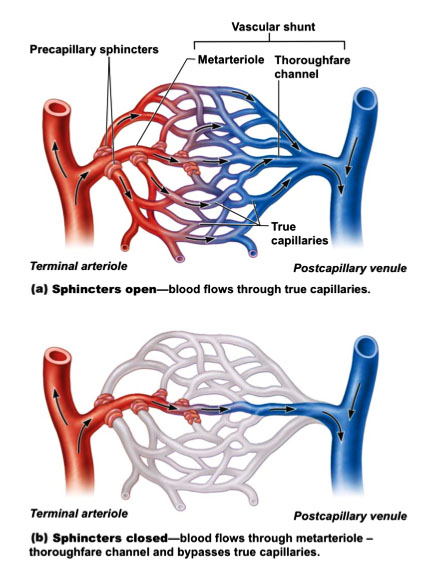
A capillary bed consists of a network of interweaving capillaries. These capillary beds connect arterioles, and venules, the smallest vessels of the venous system. In most of the capillary beds, two types of vessels are found:
- a vascular shunt
- and true capillaries
A vascular shunt connects connects an arteriole with a venule directly. These vessels do not exchange materials with the surrounding fluid. True capillaries exchange materials. The arteriole that directly connects with the capillaries is called a terminal arteriole and this arteriole feeds directly into the metarteriole. This metarteriole feeds into the thoroughfare channel and then the postcapillary venule. These vessels together make the vascular shunt.
True capillaries normally branch off the metarteriole and empty in the postcapillary venule. The root of the true capillaries is surrounded by smooth muscle cells. This is called a precapillary sphincter. It acts as a valve that regulates blood flow through the capillary bed. If the sphincters are contracted, the blood will pass through the vascular shunt, and no exchange of materials will take place. If the valves are relaxed, blood will flow through the capillaries and exchange with the surrounding cells occurs.
Postural Tachycardia Syndrome (POTS)
POTS is a hetergeneous group of disorders with similar cinlical manifestations. POTS is characterized by a combination of an abnormally high heart frequency (tachycardia) while standing (postural) and symptoms of palpitations, light-headedness, and other sensations that occur due to cerebral hypoperfusion (see symptoms and signs of presyncope)
An abnormal heart rate respons is defined as:
- Sustained heart rate increment of > 30 bpm within 10 minutes of standing or head-up tilt
- Patients aged 12-19 years require an increment of > 40 bpm
- Or a heart rate that exceeds 120 bpm
- The heart rate increment cannot be associated conditions as prolonged bed rest, or medications that diminish vascular or autonomic tone
- Symptoms of cerebral hypoperfusion and autonomic overactivity
Pathophysiology
The etiology and pathophysiology of POTS are unknown, but are likely to be heterogeneous. The syndrome is associated with deconditioning, recent viral illness, chronic fatigue syndrome and a limited or restricted autonomic neuropathy. The differential diagnosis includes conditions that cause tachycardia, such as thyrotoxicosis, inappropriate sinus tachycardia and other cardiac rhythm abnormalities, pheochromocytoma, hypoadrenalism, anxiety, dehydration, and medications (e.g., vasodilators, diuretics, and b-agonists). Many patients report that their symptoms started after a febrile illness, pregnancy, surgery, trauma or sepsis. The present thought on the pathophysiology of POTS after such an event is that POTS is an autoimmune disorder.
Epidemiology
The prevalence of POTS is not known, but estimates suggest at least 500.000 people are affected by POTS in the United States alone[28]. The syndrome is more common in women.
Clinical presentation
The orthostatic symptoms of POTS consists of light-headedness, visual blurring or tunnel vision, palpitations, tremulousness, and weakness (especially in the legs). Other symptoms include fatigue, exercise intolerance, hyperventilation, shortness of breath, anxiety, chest pain, nausea, acral coldness or pain, and concentration difficulaties and headaches. On clinical examination, in addition to the heart rate increment, pulse pressure may be reduced and acral coldness may be present. Continued standing may lead to venous prominence, cyanosis and foot swelling. A hyperadrenergic state is present in some patients who have a resting tachycardia, sweating, and tremulousness.
References
- Grubb BP. Postural tachycardia syndrome. Circulation. 2008 May 27;117(21):2814-7. DOI:10.1161/CIRCULATIONAHA.107.761643 |
test